Introduction
Many patients experience pain after surgery. In the field of anaesthesia there is a significant interest in preventing postoperative pain, but still it remains a major problem. Postoperative pain is the greatest concern and fear among surgical patients (1). Acute postoperative pain is followed by persistent postoperative pain in 3–85 per cent of patients following regular routine surgery (2). The use of opioids is central to prevent and treat pain related to surgical procedures. A prerequisite for an optimal postoperative course is effective and safe postoperative pain management (3).
Postoperative pain can have a harmful effect on circulation, respiration, and gastrointestinal function (3). This can also result in delayed or complicated wound healing (4). Mathiesen and Dahl (3) assert that intra-abdominal surgical procedures lead to a pain and reflex mediated lung function impairment. This can reduce tidal volume, functional residual capacity, and alveolar ventilation. Reduced cough reflex can cause secretion accumulation and possible pneumonia. Gastrointestinal function is affected by increased sympathetic activity by inhibiting gastrointestinal tract motility (3). Combined with pain this can lead to postoperative nausea, vomiting and ileus (3,5). Opioids can also delay gastric emptying, which triggers nausea and vomiting (5).
Oxycodone has been in clinical use since 1917 (6). In spite of long-term clinical experience with oxycodone hydrochloride / OxyNorm (OH), analgesic concentrations of OH in acute postoperative pain management have not been established. A prospective study of 23 adult patients after laparoscopic cholecystectomy showed that the minimum effective concentration and minimum effective analgesic concentration of OH are significantly higher than previously thought (7). Early pain after laparoscopic cholecystectomy appears to be a feasible method for estimating the analgesic effect of OH in acute pain management (7). Choi et al. (8) compared the efficacy of early intravenous bolus of oxycodone or fentanyl in patients undergoing laparoscopic cholecystectomy. Intravenous oxycodone bolus twenty minutes before end of surgery relieved immediate postoperative pain significantly better than fentanyl (8). Oxycodone was, in a randomised double-blind study, found to provide better analgesia when given immediately after operation, but tended to have more side-effects (9). A systematic updated review of pain management after laparoscopic cholecystectomy recommends that opioid analgesia are to be avoided if possible, due to significant side-effect profiles (10).
This study was requested as a quality improvement project for the anaesthesia department of Stavanger University Hospital. According to anaesthetic guidelines for laparoscopic cholecystectomy at Stavanger University Hospital, OH should be administered towards the end of anaesthesia. There were suspected varying practices regarding intraoperative timing of OH administration among anaesthesiologists and nurse anaesthetists. This quality improvement project was therefore requested to explore the practice of intraoperative timing of OH administration and need for postoperative OH. In spite of thorough literature searches, we have not found this described in previous research. New knowledge and adapted practice can contribute to potentially optimise patient treatment through increased patient satisfaction, less pain and postoperative complications, as well as faster course of day-case surgery.
The aim of the study was to determine to which extent intraoperative timing of OH administration affects the need for postoperative OH during target-controlled propofol and remifentanil infusion (TCI) for patients undergoing laparoscopic cholecystectomy.
Method
Study design and patient population
We designed a retrospective observational study adhering to the “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines. Our study of medical records consisted of anaesthetic journals and postoperative journals from one hundred patients undergoing laparoscopic cholecystectomy.
Inclusion/exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| > 18 years of age | Inhalation anaesthesia |
|
Elective day-case laparoscopic cholecystectomy TCI with propofol and remifentanil (TCI models; Marsh for propofol and Minto for remifentanil) ASA 1-2 |
Medicine use that could interfere with OH MCI (Manually-controlled infusion) Having not received all the anaesthetic agents and medications following the anaesthetic guideline for laparoscopic cholecystectomy (ref. supplement 1) Having received postoperative analgesics that could interfere with OH (this did not apply to standard postoperative analgesics such as paracetamol and diclofenac) |
Data collection consists of all laparoscopic cholecystectomies conducted at Stavanger University Hospital between September 2015 and December 2016, during which period there were no changes in operating method or anaesthetic guideline for laparoscopic cholecystectomy. Inclusion and exclusion criteria are listed in Box 1. Briefly, we included adult patients ASA 1-2 (American Society of Anaesthesiologists Physical Status Classification System) (11) who underwent elective day-case surgery using TCI and following the anaesthetic guideline for laparoscopic cholecystectomy (ref. supplement 1). Premedication consisted of oral paracetamol. OH was the only opioid in the guideline and was to be administered at the end of anaesthesia in addition to Marcain for wound infiltration. Additional medications given intraoperatively to all patients were dexamethasone, parecoxib, and ondansetron. OH was the only opioid agent given for postoperative pain treatment.
Conduct of the study
The data collection was conducted by two persons who separately reviewed anaesthetic journals and associated postoperative journals from patients after laparoscopic cholecystectomy. A total of 546 patients satisfied the study’s inclusion criteria. Of these, 399 patients were excluded due to the pre-specified exclusion criteria (Box 2). The main reason for exclusion was use of inhalation anaesthesia. Furthermore, 47 patients were excluded due to missing anaesthetic or postoperative journals, unclear writing on records or incorrect registration of total intravenous anaesthesia (TIVA) agents.
| Number (N) | |
|---|---|
|
Inhalation anaesthesia Medicine use that could interfere with OH MCI No local anaesthesia Age Received postoperative analgesics that could interfere with OH |
308 29 55 3 1 3 |
Other reasons |
47 |
| Total | 446 |
Data handling
In compliance with requirements from the Data Protection Officer at Stavanger University Hospital, no data from patient records were copied, printed or written on paper. Research data was recorded and stored anonymously on an encrypted memory chip.
Variables
Information registered for each patient was age, sex, height, weight, date and time of start of TCI, date and time of end of TCI, total dose of propofol and remifentanil, time and amount for administration of OH intra- and postoperatively, other analgesics that were administered postoperatively, and pain score (Visual Analogue Scale – VAS).
Total dose of both propofol and remifentanil given to each patient was automatically registered in the anaesthetic journal. This also applies to time of anaesthesia recorded from TCI start to TCI end. Amount of OH administered postoperatively was manually registered in the postoperative journals.
Despite long clinical experience with OH, neither the minimum effective plasma concentration nor the minimum effective analgesic concentration have been established (7). Some articles describe that maximum plasma concentration of OH is reached after about twenty minutes with intravenous (iv) administration (8,12,13). Other studies show that OH iv provides rapid pain relief after about five to eight minutes (14). Pharmacists at the pharmaceutical company Mundipharma stated that OH has maximum effect immediately after iv administration of OH (personal communication). Based on the uncertainty concerning the concentration of OH, we have in this study defined administration of OH towards the end of anaesthesia as during the last twenty minutes of TCI.
The timing of OH administration intraoperatively was split into three categories. First, we separated those patients who received OH towards the end of TCI from those who did not. Furthermore, to isolate those who received OH only towards the end of TCI (anaesthetic guideline), these were placed in a separate group (Box 3).
| Category | Name | Description |
|---|---|---|
| 1 | All times | OH administered towards the end in addition to the introduction/maintenance of TCI |
| 2 | Not end | OH administered in the beginning/during the maintenance of TCI, but NOT towards the end |
| 3 | End only | OH administered ONLY towards the end of TCI (last 20 min) |
Statistics
The collected research data was analysed using IBM SPSS Statistics version 24, unless otherwise stated. Descriptive statistics were presented as median and interquartile range (IQR) and as mean and standard deviation (SD) for continuous data, and as counts and percentages for categorical data. Box plots were used for illustration. Median values were compared non-parametrically using Kruskal-Wallis tests.
For comparison of mean values of OH postoperatively and the combined intra- and postoperative doses of OH between groups of OH administration timing, we used regression analysis. As the outcomes were skew distributed, analyses were performed with generalised linear models with log link (15) and using robust (sandwich) estimates of standard errors. Results are presented as marginal means (i.e., average adjusted predictions (16)) with 95 per cent confidence intervals (CI), with and without adjustment for patient and operational variables. The effects of OH timing categories were tested using Wald Chi-square tests. The regression analyses were performed in Stata version 16 with function poisson applying option vce (robust), and with functions margins and test. P-values less than 0.05 were considered statistically significant.
The sample size of N = 100 was chosen based on the rule of thumb of ten observations per variable in a linear regression i.e. to allow for flexibility and adjustments in the comparisons of timing groups.
Ethical considerations
This study was approved by the Head of the Anesthesia Department, the Research Department and the Data Protection Officer at Stavanger University Hospital. According to Stavanger University Hospital’s research policy, access was granted to anaesthetic journals, associated postoperative journals and patients medication records. The Regional Committees for Medical and Health Research Ethics considered the project as a quality improvement project and therefore did not require informed consent from individual patients, ref. 603276.
Results
The characteristics of the 100 included patients are given for the entire selection and individually for each category (1–3) in Table 1. All patients in the study were assigned an ASA classification 1 or 2. The majority of the sample were women (N = 71). The youngest patient was 19 years old and the oldest was 70 years old. The mean age was 44 (SD = 13).
| Timing of OH intraoperatively | ||||
|---|---|---|---|---|
| Total | Category 1 All times | Category 2 Not end | Category 3 End only | |
| (n = 100) | (n = 20) | (n = 66) | (n = 14) | |
| Patient characteristics | ||||
| Male sex, n (%) | 29 (29%) | 3 (15%) | 22 (33%) | 4 (29%) |
| Age (years) | 43.0 (34.0, 52.8) | 40.5 (35.5, 51.8) | 45.5 (33.8, 55.5) | 37.0 (33.0, 49.5) |
| Observed weight (kg) | 76.0 (67.0, 90.0) | 77.5 (66.3, 90.0) | 75.5 (68.8, 91.3) | 77.5 (60.3, 90.0) |
| CBW (kg) | 70.0 (66.0, 82.8) | 68.5 (65.3, 75.5) | 74.0 (67.0, 85.0) | 67.5 (60.3, 82.0) |
| BMI > 30, n (%) | 25 (25%) | 7 (35%) | 15 (23%) | 3 (21%) |
| Operation variables | ||||
| TCI start – end (minutes) | 90.0 (70.0, 110.0) | 95.0 (62.5, 125.0) | 90.0 (70.0, 110.0) | 70.0 (57.5, 90.0) |
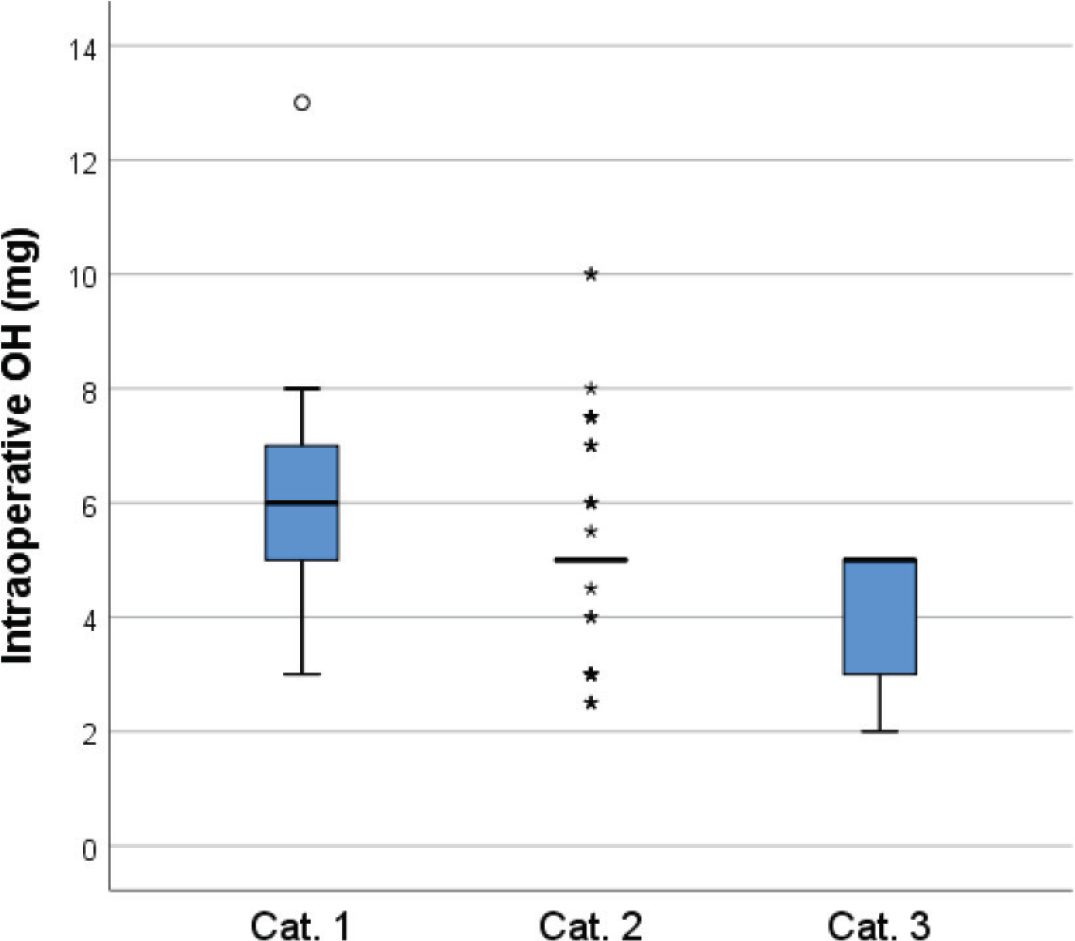
| Total OH dose intraoperatively (mg) | 5.0 (5.0, 6.0) | 6.0 (5.0, 7.0) | 5.0 (5.0, 5.1) | 5.0 (2.9, 5.0) |
| Total remifentanil dose (ml) | 24.9 (17.6, 36.4) | 24.4 (16.6, 38.8) | 26.0 (18.8, 39.0) | 17.4 (15.3, 31.9) |
| Total propofol dose (ml) | 86.5 (66.1, 116.1) | 81.1 (59.7, 116.1) | 90.6 (70.9, 115.2) | 79.5 (54.3, 127.5) |
| Outcomes | ||||
| Total OH dose postoperatively (mg) | 5.0 (2.5, 8.0) | 5.0 (2.3, 7.5) | 5.5 (2.5, 10.0) | 5.0 (2.4, 7.5) |
| Total OH dose intra- and postoperatively (mg) | 10.3 (7.5, 13.0) | 10.0 (9.3, 12.9) | 10.5 (7.5, 15.3) | 8.8 (5.4, 12.5) |
Descriptives given as median (interquartile range), unless otherwise specified. Abbreviations: OH Oxycodone hydrochloride, CWB Corrected body weight, BMI Body mass index, TCI Target-controlled infusion.
Twenty (20%) of the patients received OH towards the end of TCI in addition to the introduction of and/or maintenance of TCI. Most patients (N = 66; 66%) received OH at the beginning and/or during the maintenance of TCI, but not towards the end of TCI. Only 14 patients (14%) received OH only towards the end of TCI.
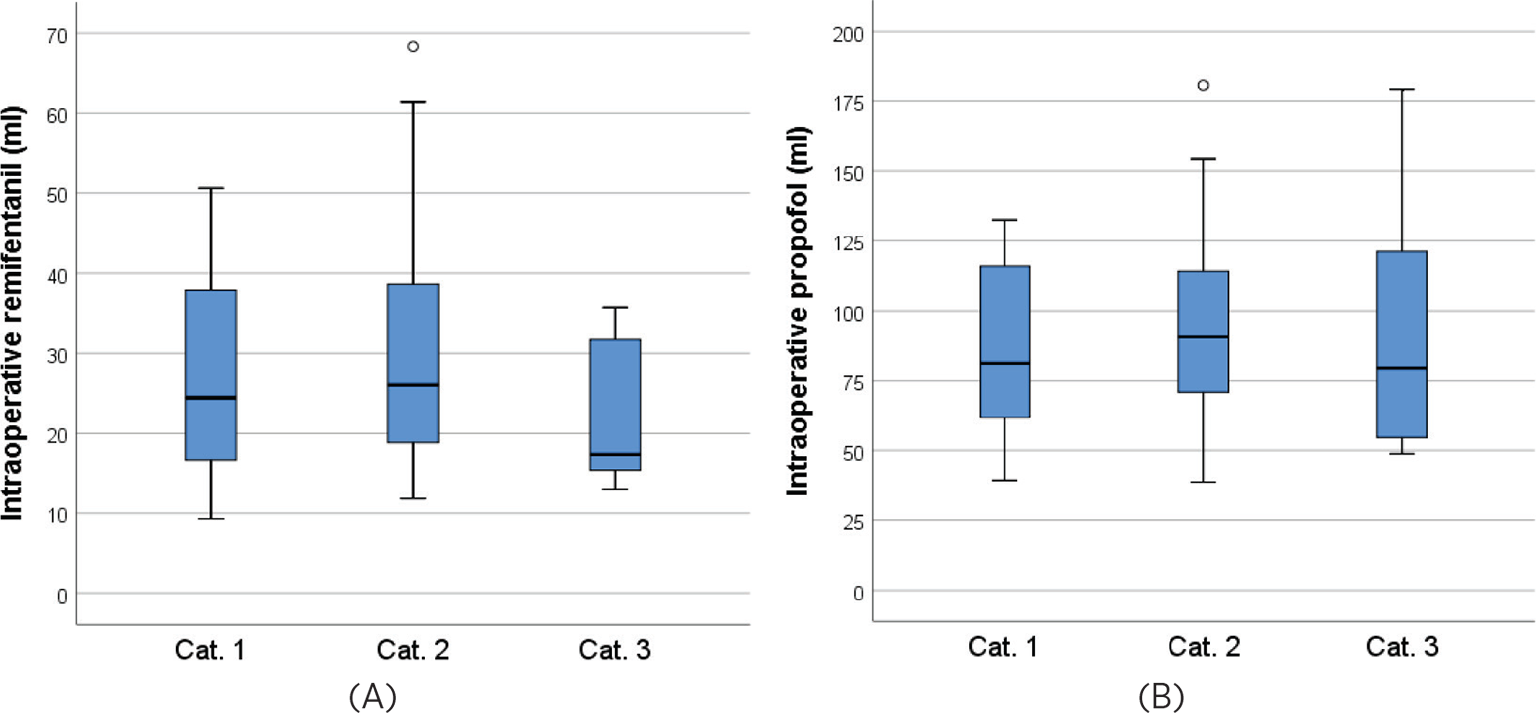
The doses of remifentanil and propofol intraoperatively are further illustrated in Figure 1. There is a tendency that the patients who received OH only at the end of TCI also received lower medication doses, however the differences between groups were not statistically significant (Kruskal-Wallis tests, p = 0.080 and p = 0.70 for remifentanil and propofol, respectively).
There were, however, statistically significant differences in total dose OH administered intraoperatively between the groups for timing of intraoperative OH: Kruskal-Wallis p = 0.001 (Figure 2).
Intraoperative timing of OH administration, need for postoperative OH, and combined total amounts of OH
The observed distributions of total postoperative and combined intra- and postoperative doses of OH administered for patients in different categories for timing of OH administration are displayed in Figure 3. We found no statistically significant differences in median OH administered postoperatively between the groups for timing of OH intraoperatively (Kruskal-Wallis p = 0.19). The same was the case for the combined intra- and postoperative doses of OH (p = 0.12).
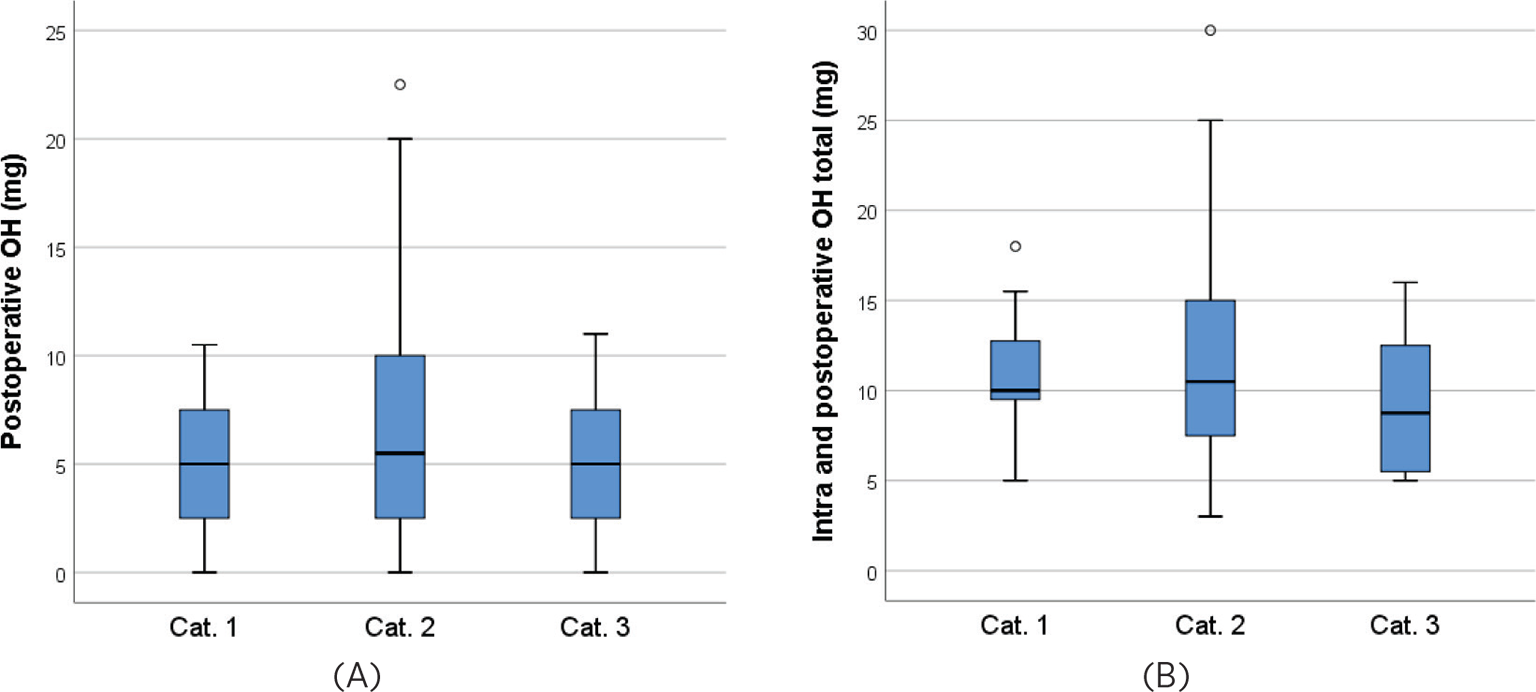
Observed means (SD) of postoperatively administered OH in mg for categories 1–3 were 4.7 (3.1), 6.9 (5.1), and 4.7 (3.4), respectively; with an overall p-value for the test of equal groups of 0.032, see Table 2 which also presents confidence intervals. Adjusting for age, gender, weight, operating time and medications given during operation did not in total affect the results substantially, and the differences were still statistically significant (p = 0.012). Pairwise comparisons in the fully adjusted model indicated differences between categories 1 and 2 (p = 0.017) and between categories 2 and 3 (p = 0.042), but not between categories 1 and 3 (p = 0.83).
In total during the intra- and postoperative course, observed means (SD) of OH administered were 11.0 (2.9), 12.1 (5.5), and 9.0 (3.5) mg for patients in categories 1–3, respectively, and before adjustment these differences were statistically significant (p = 0.039). The marginal means were only slightly affected by adjusting for patient and operational variables, however the group differences were no longer statistically significant (p = 0.057).
| Category 1 All times | Category 2 Not end | Category 3 End only | ||
|---|---|---|---|---|
| Outcomes/adjustments | Marginal mean (95% CI) | Marginal mean (95% CI) | Marginal mean (95% CI) | p |
| Total OH dose
postoperatively (mg) |
||||
| Unadjusted | 4.7 (3.3, 6.0) | 6.9 (5.7, 8.1) | 4.7 (3.0, 6.4) | 0.032 |
| Adjusted for age, gender, weight | 4.5 (3.3, 5.8) | 7.0 (5.8, 8.2) | 4.6 (2.9, 6.4) | 0.014 |
| + adjusted for operating time* | 4.5 (3.3, 5.7) | 6.9 (5.7, 8.1) | 5.0 (3.1, 6.9) | 0.021 |
| + adjusted for medication given intraoperatively** | 4.7 (3.4, 6.0) | 7.0 (5.8, 8.2) | 4.4 (2.6, 6.2) | 0.012 |
| Combined total OH dose intra- and postoperatively (mg) | ||||
| Unadjusted | 11.0 (9.8, 12.3) | 12.1 (10.7, 13.4) | 9.0 (7.2, 10.8) | 0.039 |
| Adjusted for age, gender, weight | 11.0 (9.8, 12.3) | 12.1 (10.8, 13.3) | 9.0 (7.2, 10.8) | 0.041 |
| + adjusted for operating time* | 10.8 (9.6, 12.1) | 12.0 (10.8, 13.2) | 9.5 (7.6, 11.5) | 0.13 |
| + adjusted for remifentanil and propofol doses | 10.9 (9.7, 12.0) | 12.1 (10.9, 13.3) | 9.1 (7.1, 11.1) | 0.057 |
* Operating time defined as TCI start to end.
** Total propofol dose, total dose of remifentanil, total dose of OH intraoperative.
Marginal means; expected dose (with regard to the adjustment factors), however all patients were (hypothetically) administered OH intraoperatively at times as defined by, respectively, category 1–3.
Discussion
The results support our assumptions that there is a relationship between the intraoperative timing of OH and the need for OH postoperatively. Mean total postoperative doses of OH for the different groups were statistically significantly different, also after adjusting for confounding factors. Median values were more similar between the categories. The patients in category 2 (not end) received higher doses of OH postoperatively compared to the other patients. Combined total dose of OH intra- and postoperatively for patients that received OH only towards the end of TCI was substantially lower than for patients in other categories, however the evidence is weaker for this effect.
Although the anaesthetic guideline says to give OH only towards the end of TCI, this was done in merely 14 per cent of the included operations. Presumably, because anaesthesia must be customised to the individual patient. The anaesthetic guideline is to be considered as a recommendation, not as standard. It is also possible that the time from administration of OH to the end of surgery has taken longer than expected, resulting in a larger number of patients in category 1 (all times) and 2 (not end). Even so, one would expect a higher proportion of operations with practice that were in compliance with the guideline. Our results indicate a lack of anchoring of the guidelines within the intended users.
The results are clinically relevant and indicate that it may be beneficial to avoid administration of OH before the end of TCI, despite existing uncertainty concerning the metabolism of OH (7,12–14). Our findings support previous research regarding intraoperative timing administration of opioids. A systematic review studied the role of timing analgesia, i.e., compared preoperative versus intraoperative or postoperative initiation of analgesia. They found that preventive administration of opioids does not improve postoperative pain control (17). Many studies have described the complexity of pain after laparoscopic cholecystectomy and that analgesic treatment should be managed using a multifaceted opioid-sparing analgesic regimen. Prophylactic pain treatment with opioids is not recommended due to significant side-effect profiles (9,10). An overall minimised opioid consumption is beneficial for patients. Kehlet (5), among others, have shown that postoperative opioids are a reliable predictor of PONV (postoperative nausea and vomiting).
Remifentanil has a very short duration of action. Analgesics for postoperative pain relief must be administered sufficiently before discontinuing the infusion, so that therapeutic efficacy is achieved before the opioid effect of remifentanil ceases (18). There is still uncertainty about the impact time and the maximum analgesic effect of OH (7,12–14). The results from this study showed that patients in both category 1 (all times) and category 2 (not end) received significantly higher doses of remifentanil compared to patients in category 3 (end only). Common features for these patients were that OH was administered earlier in the intraoperative phase. It is possible that it was done in hopes of reducing the dose of remifentanil and thereby avoiding the development of opioid-induced hyperalgesia (OIH) and acute opioid tolerance (AOT). To the contrary, the results showed that these patients received a much higher dose of remifentanil compared to the patients that received OH only towards the end of TCI. In light of this, it may seem most beneficial to administer OH only towards the end of TCI, as these patients received the least dose of remifentanil, as well as the least dose of OH in total. At the same time, one cannot know for sure whether this can be explained by timing of OH alone, as total TCI-duration for patients in category 3 (end only) was substantially shorter than for the other categories. Other factors, such as the patient pain thresholds and postoperative pain assessment, are also likely to influence the outcome, without this being revealed in the present study.
OIH and AOT related to remifentanil-based anaesthesia still entail a great deal of uncertainty (19,20). Previous studies have not shown strong indications supporting the need to reduce the dose of remifentanil or to use modalities that prevent OIH and AOT (21,22). However, co-administered anaesthetics, such as propofol, and using TCI model seem to be helpful to modulate the development of OIH and AOT (22). We therefore chose to include TCI with propofol and remifentanil in the study, while manually-controlled infusion (MCI) was excluded. For the same reason, inhalation anaesthesia was excluded from the study (23).
Although the results of this study can only be considered to be valid for patients that are undergoing the same surgical procedure with the same anaesthetic method and agents, it is likely that the result can be transferred to other types of surgical procedures at Stavanger University Hospital. This provides the foundation for further research. Randomised controlled trials will, to an even greater extent, be able to compare the categories for timing of OH administration against each other and measure their effects.
Strengths and limitations
Our study has several strengths. Observational studies that require informed consent for use of data from medical records is reported to introduce selection bias. Significant differences between participants and non-participants may threaten the validity of results (24). In this study this was avoided through exemption from obtaining consent (approved by the Data Protection Officer). Meaning, anaesthetic journals and associated postoperative journals from all patients that met the criteria for inclusion and exclusion were included in the study. The reliability of the study is enhanced by two individuals separately reviewed anaesthetic journals and associated postoperative journals and controlled the data entry, as well as the data being recorded over a time period of 16 months, which ensured that individual factors of the anaesthesia personnel did not affect the result.
We applied, according to Stavanger University Hospital’s research policy, to the Data Protection Officer at Stavanger University Hospital for permission to access all patient medication lists. Patient records related to medicine use that could affect pain levels and outcomes were excluded from the study. Grapefruit and St. John’s wort affects the plasma concentration of OH (25). Unfortunately, in this retrospective study, it was not possible to rule out whether patients had used this preoperatively, as it is rarely recorded in a journal. Anaesthetic journals and postoperative journals registered with other anaesthetic agents or medications than the current procedure (ref. supplement 1) were excluded from the study. Some of the patients in the study had received OH orally instead of or in addition to OH intravenously. These patients were included in the sample. The oral OH dose was converted to intravenous OH dose according to guidelines at Stavanger University Hospital.
To exclude relevant interactions between TIVA agents (propofol and remifentanil) and their effect on OH, interaction analyses were conducted in collaboration with a pharmacist. The analyses were conducted in three online encyclopaedias: felleskatalogen.no, interaksjoner.no and drugs.com. In the first two, we found no relevant interactions between these agents. The interaction analyses conducted on drugs.com showed a total of 13 articles, with non-relevant results for this study. Furthermore, changed pharmacokinetics in obese patients leads to a risk of overdose (26). Obesity is defined as BMI > 30 (27). In this study analyses were done with observed weight. However, we would like to point out that the same analyses have been carried out with corrected body weight, which gave very similar results.
The study also has limitations. Information bias may have occurred due to errors related to the registration of journal data. Administered doses of propofol and remifentanil were automatically registered in anaesthetic journals. Time of administration and amount of OH were manually registered and errors may have occurred both intra- and postoperatively. Unfortunately, we cannot exclude these aspects, however, this should be taken into account for the results of the study.
Of the 499 journals that were readily assessed, 399 (80%) were excluded due to pre-specified criteria. Thus, if we assume the same would be the case for the 47 journals with unclear information, we will have lost 9–10 patients that were in the target group, which would amount to about ten per cent. We have no indication that these 9–10 patients would be systematically different from the one hundred included patients, and regard our sample as representative for the group defined by the inclusion/exclusion criteria. The relatively small sample size, especially the small number of patients in category 3 (only end), makes the study susceptible to both type I and type II errors, thus the results must be interpreted with caution and should be validated and elaborated on in a larger study.
Conclusion
In our study, intraoperative timing of OH administration seems to affect the need for OH postoperatively after laparoscopic cholecystectomy with TCI (propofol and remifentanil) as the anaesthetic method. Patients who did not receive OH towards the end of TCI were associated with a greater need for OH postoperatively. This suggests that to reduce the total amount of OH, it may be beneficial to administer OH only towards the end of TCI. Given the relatively small sample size, our results should be carefully interpreted. Further research is needed to confirm our findings.
References
- 1. Small C, Laycock H. Acute postoperative pain management. Brit J Surg. 2020;107:70–80. https://doi.org/10.1002/bjs.11477
- 2. Gulur P, Nelli A. Persistent postoperative pain: mechanisms and modulators. Curr Opin Anesthesiol. 2019;32(5):668–73. https://doi.org/10.1097/ACO.0000000000000770
- 3. Mathiesen O, Dahl JB. Postoperativ smertebehandling. In: Rasmussen LS, Steinmetz J, eds. Anæstesi. København: FADL’s Forlag; 2014. p. 281–293.
- 4. Van Boekel RLM, Warle MC, Nielen RGC, Vissers KCP, van der Sande R, et al. Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 2019;269:856–65. https://doi.org/10.1097/SLA.0000000000002583
- 5. Kehlet, H. Postoperative pain, analgesia, and recovery-bedfellows that cannot be ignored. Pain. 2018;11–19. https://doi.org/10.1097/j.pain.0000000000001243
- 6. Falk E. Eukodal, ein neues Narkotikum. Munchen Med Wochen. 1917;20:381–84.
- 7. Kokki M, Broms S, Eskelinen M, Rasanen I, Ojanperä I, et al. Analgesic concentrations of oxycodone – a prospective clinical PK/PD study in patients with laparoscopic cholecystectomy. Basic Clin Pharmacol Toxicol. 2012;110:469–75. https://doi.org/10.1111/j.1742-7843.2011.00839.x
- 8. Choi YJ, Park SW, Kwon HJ, Moon YJ, Lee YM. Efficacy of early intravenous bolus oxycodone or fentanyl in emergence from general anaesthesia and postoperative analgesia following laparoscopic cholecystectomy: A randomized trial. J Int Med Res. 2015;43:809–18. https://doi.org/10.1177/0300060515594194
- 9. Koch S, Ahlburg P, Spangsberg N, Brock B, Tønnesen E, et al. Oxycodone vs. fentanyl in the treatment of early post-operative pain after laparoscopic cholecystectomy: a randomised double-blind study. Acta Anaesthesiol Scand. 2008;52:845–50. https://doi.org/10.1111/j.1399-6576.2008.01643.x
- 10. Barazanchi AWH, MacFater WS, Rahiri JL, Hill AG, Joshi GP. Evidence-based management of pain after laparoscopic cholecystectomy: a PROSPECT review update. Brit J Anaesth. 2018;121:787–803. https://doi.org/10.1016/j.bja.2018.06.023">
- 11. American Society of Anesthesiologists. ASA physical status classification system, 2020 [Internet]. Available from: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
- 12. Pöyhiä R, Olkkola KT, Seppälä T, Kalso E. The pharmacokinetics of oxycodone after intravenous injection in adults. Brit J Clin Pharmaco. 1991;32:516–18. https://dx.doi.org/10.1111/j.1365-2125.1991.tb03942.x
- 13. Kalso E. Oxycodone. J Pain Symtom Manag. 2005;29:47–56. https://doi.org/10.1016/j.jpainsymman.2005.01.010
- 14. Pergolizzi JV, Seow-Choen F, Wexner SD, Zampogna G, Raffa RB, et al. Perspectives on intravenous oxycodone for control of intraoperative pain. Pain Pract. 2015;16:924–34. https://doi.org/10.1111/papr.12345
- 15. Gould W. Use poisson rather than regress; tell a friend. Texas: The STATA Blog; 2011 [cited Feb 3, 2017]. Available from: https://blog.stata.com/2011/08/22/use-poisson-rather-than-regress-tell-a-friend/
- 16. Stata. Margins – marginal means, predictive margins, and marginal effects [Internet]. Texas: Stata; nd [cited Feb. 3, 2017]. Available from: https://www.stata.com/manuals/rmargins.pdf
- 17. Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anaesthesiol. 2002;96:725–41. https://doi.org/10.1097/00000542-200203000-00032
- 18. Felleskatalogen. Ultiva [Internet]. Oslo: Felleskatalogen; 2016 [cited Jan 3, 2017]. Available from: http://www.felleskatalogen.no/medisin/ultiva-glaxosmithkline-564913
- 19. Angst MS. Intraoperative use of remifentanil for TIVA: intraoperative pain, acute tolerance, and opioid-induced hyperalgesia. J Cardiothor Vasc An. 2015;29:16–22. https://doi.org/10.1053/j.jvca.2015.01.026
- 20. Chu LF, Angst MS, Clark D. Opioid-induced hyperalgesia in humans. Clin J Pain. 2008;24:479–96. https://doi.org/10.1097/ajp.0b013e31816b2f43
- 21. Angst MS, Clark D. Opioid-induced hyperalgesia. A qualitative systematic review. Anaesthesiol. 2006;104:570–87. https://doi.org/10.1097/00000542-200603000-00025
- 22. Kim SH, Stoicea N, Soghomonyan S, Bergese SD. Intraoperative use of remifentanil and opioid induced hyperalgesia/ acute opioid tolerance: systematic review. Front Pharmacol. 2014;5:1–9. https://doi.org/10.3389/fphar.2014.00108
- 23. Schraag S, Pradelli L, Alsaleh AJO, Bellone M, Ghetti G, et al. Propofol vs. inhalational agents to maintain general TCI in ambulatory and in-patient surgery: a systematic review and meta-analysis. BMC Anaesthesiol. 2018;18:162. https://doi.org/10.1186/s12871-018-0632-3
- 24. Kho ME, Duffet M, Willison DJ, Cook DJ, Brouwers MC. Written informed consent and selection bias in observational studies using medical records: systematic review. Brit Med J. 2009;338:b866. https://doi.org/10.1136/bmj.b866
- 25. Felleskatalogen. OxyNorm [Internet]. Oslo: Felleskatalogen; 2015 [cited Dec 14, 2016]. Available from: https://www.felleskatalogen.no/medisin/oxynorm-mundipharma-592269
- 26. Casati A, Putzu M. Anaesthesia in the obese patient: Pharmacokinetic considerations. J Clin Anaesth. 2005;17:134–45. https://doi.org/10.1016/j.jclinane.2004.01.009
- 27. Norsk elektronisk legehåndbok. Overvekt [Internet]. Tiller: Norsk helseinformatikk; 2016 [cited Mar 20, 2017]. Available from: https://legehandboka.no/handboken/kliniske-kapitler/endokrinologi/tilstander-og-sykdommer/overvekt/overvekt/
- 28. Absalom A, Struys MMRF. An overview of TCI & TIVA. 3rd ed. Belgie: Academia press; 2019.
Supplements
| Premedication | Paracetamol 1–1.5 g |
| Introduction of anaesthesia | TIVA with propofol and remifentanil Rokuronium 0.6 mg/kg iv |
| Maintenance of anaesthesia | TIVA with propofol and remifentanil Rocuronium 0.15 mg/kg iv |
| Additional medications given intraoperatively to all patients | Dexamethasone 8 mg iv Parecoxib 40 mg iv Ondansetron 4 mg iv |
| End of anaesthesia | Oxycodone hydrochloride (OH) 5 mg iv Marcain 0.5% up to 20 ml for wound infiltration Robinul-Neostigmine (0.5 + 2.5 mg/ml) 1 ml |